HOME > The principle of desalination
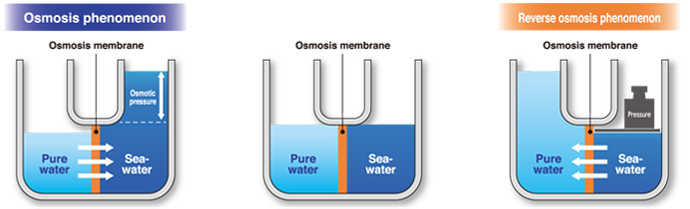
Osmotic pressure
Membranes allowing the passage of water are called gosmosishmembranes. Such membranes are found in nature, e.g., egg membranes and human skin. Cellulose acetate membranes were manufactured and commercialized some 40-50 years ago.These membranes, however, are not widely accepted due to poor chemical and physical resistance. The newest membraines are made of engineering plastics and are highly resistant to chemical and biological agents.
Osmosis membrane
When seawater and pure water are separated by a semipermeable membrane, the water flows in the direction towards the seawater(higher concentration)through the membrane. This natural process is termed gosmosis.h Osmostic pressure is the force driving the pure water towards the seawater through the membrane, induced by the difference between concentrations of solution across the membrane. The pressure is as high as 24kg/cm2.
Reverse osmosis
Revers osmosis is the forced passeage of water through a membrane against the natural osmotic pressure. This is accomplished by applying counterpressure against the osmostic pressure. A membrane for this purpose is called a greverse osmosis(RO)hmembrane. RO desalination units produce potable water from seawater under applied pressures of up to 55-68kg/cm2.Desalination units incorporating membranes of the latest technology are now widely applied to the production of potable water from seawater, saline water, or water containing chemicals or other matter rendering it undrinkable.
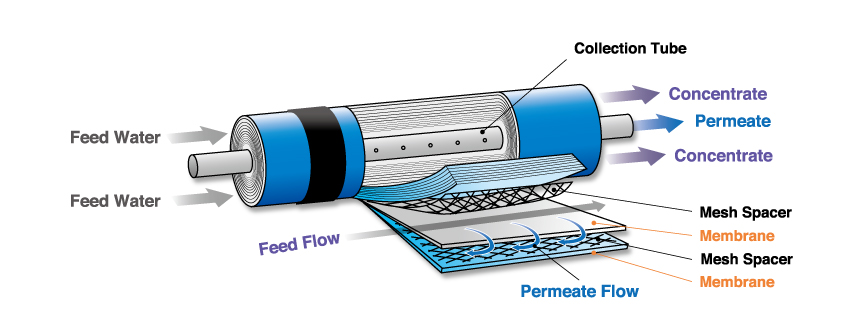
This is the interior of the gspiral woundh RO memblane. It is construted of lage thin sheets. These sheets are sealed to from envelopes enclosing the permeate collector whitch is backed with permeate spacer material. One end of each memblane envelopes is connected to a central perforated tube. There envelopes are then rolled up to from a spiral wound module enclosed by FRP. Mesh spacers are packed between membrane envelopes to allow seawater or brackish water to pass.
Typical RO membranes arre spiral-shaped in sizes of either 4~40h or 8~80h. They are enclosed in pressure vessels housing up to six membranes each. High pressure is appliced to the vessels to permeate water through the membranes. Brine is discharged from the outlet ends of the modules. The productivity of the permeate varies depending on the raw water concentration. 20-40% permeate(typically 33%)can be obtained from seawater and 50-90% from backish water.





